*Andrew W. was dosed with ROCTAVIAN in 2018 as a clinical study participant.
*Andrew W. was dosed with ROCTAVIAN in 2018 as a clinical study participant.
Andrew W., dad, husband, runner, and member of the hemophilia A community*
*At approval, ROCTAVIAN had the longest and largest Phase 3 clinical study in severe hemophilia A.
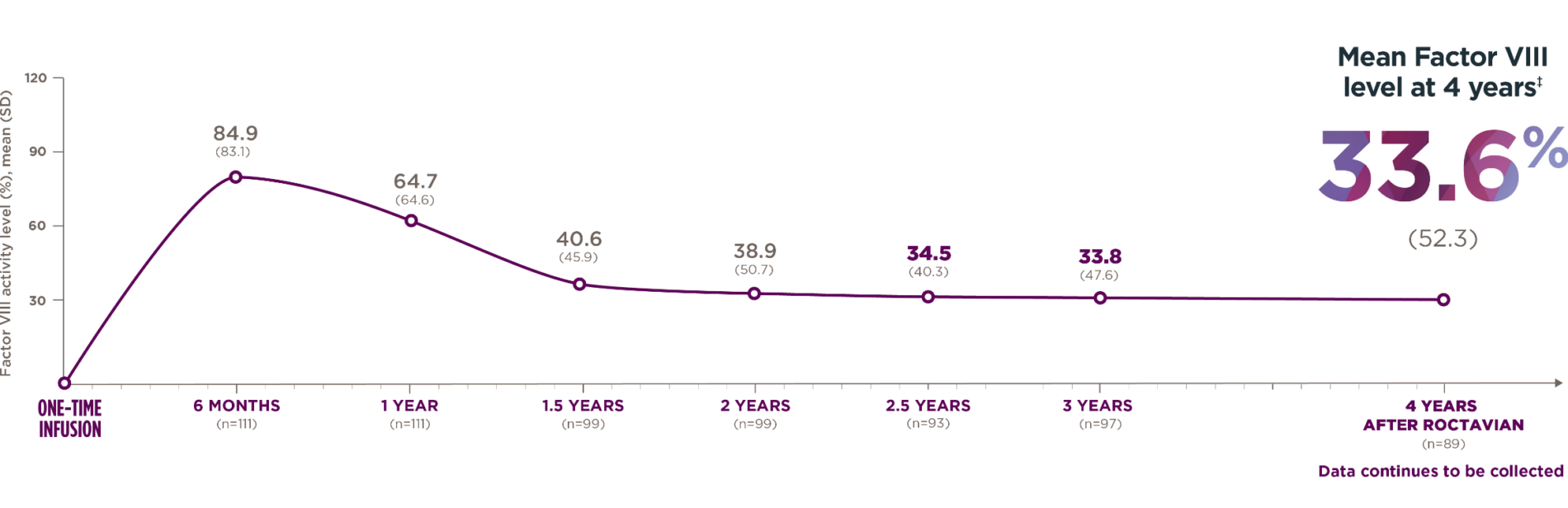
*The 4-year follow-up period began 5 weeks or more after administration and consisted of a median follow-up of 4 years with a range of 1.7 to 4.6 years.
†Standard deviation (SD) shows how spread out the data are from the average.
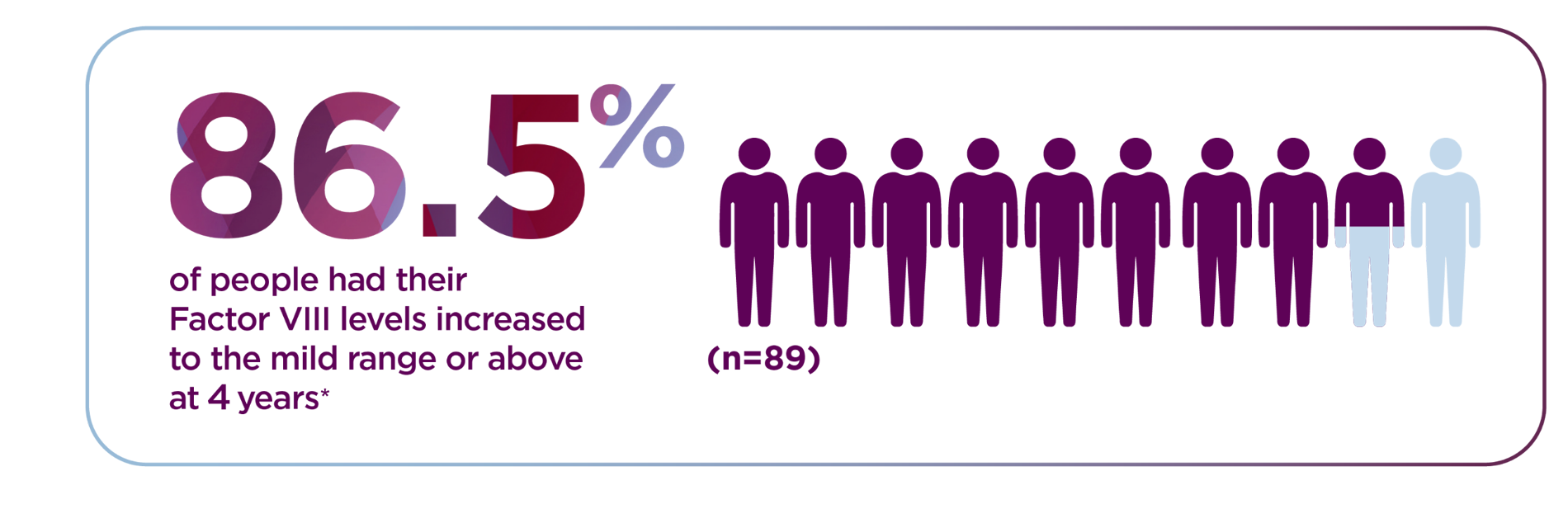
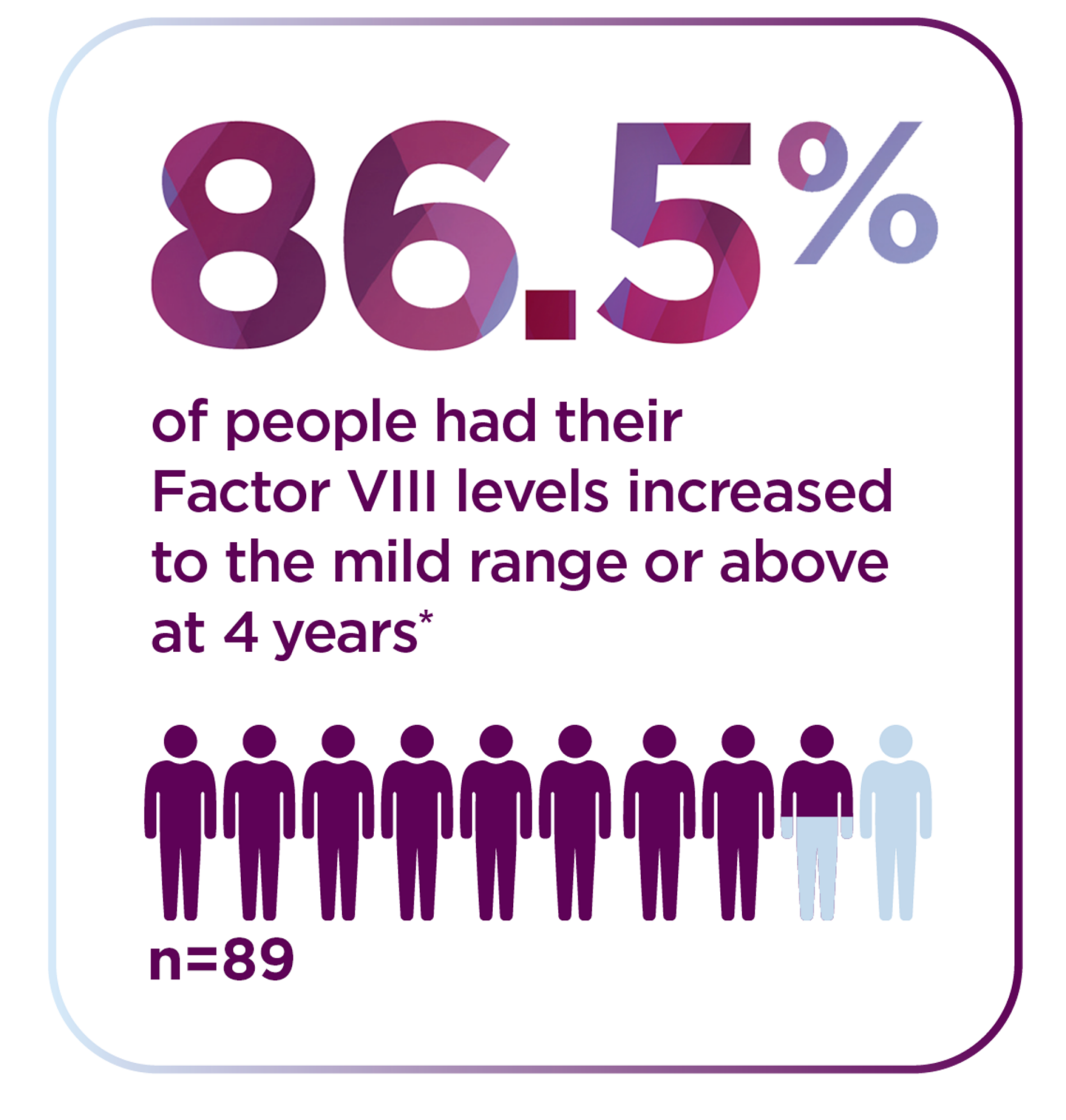
*The World Federation of Hemophilia defines mild range as 5% to less than 40%.
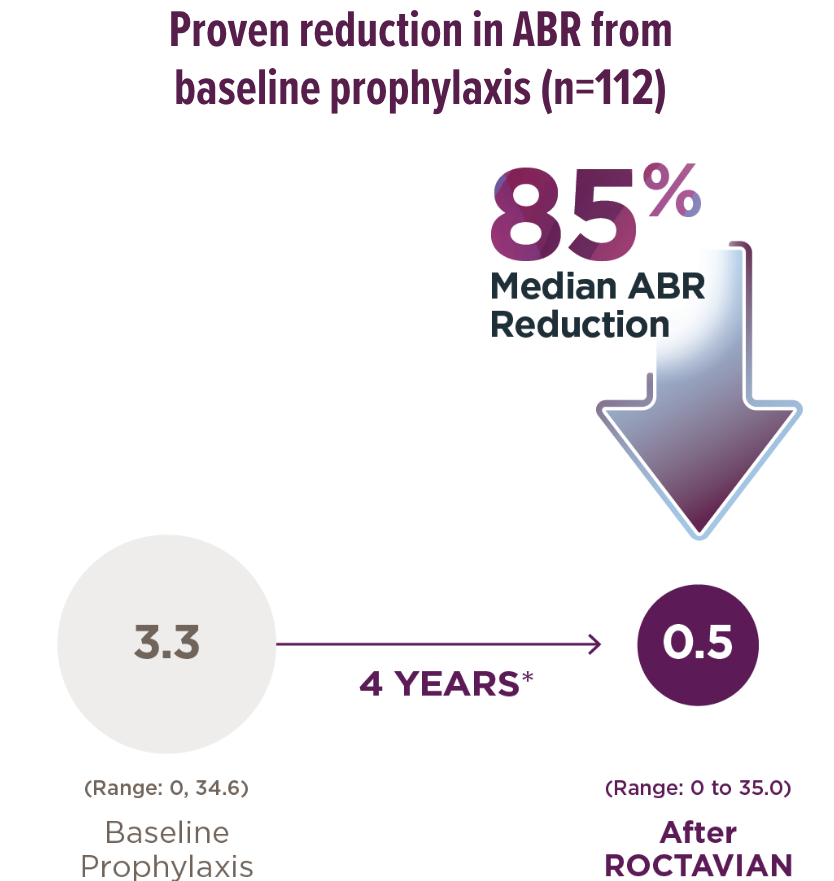
*The 4-year follow-up period began 5 weeks or more after administration and consisted of a median follow-up of 4 years with a range of 1.7 to 4.6 years. Median is the middle number in a list of numbers arranged from smallest to largest.
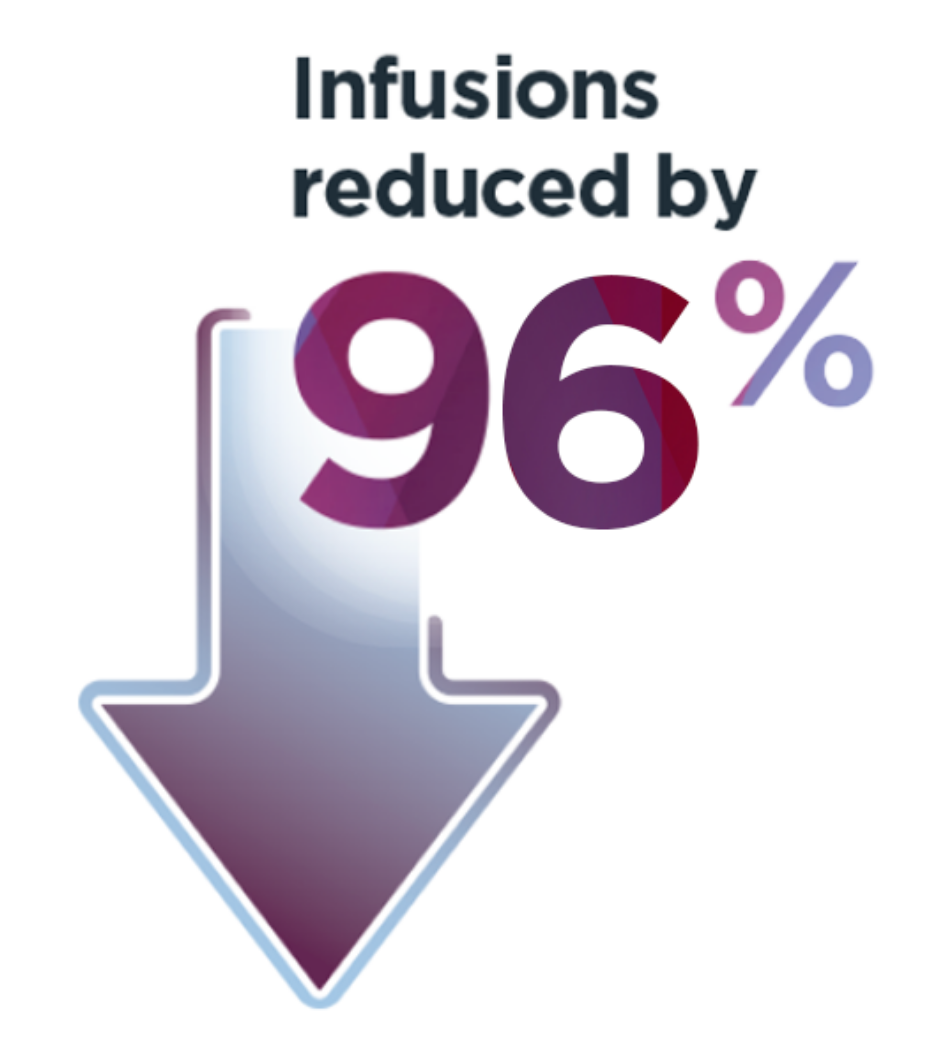
†18 of 112 people (16%) returned to continuous prophylaxis after ROCTAVIAN, with a median start time at 963 days with a range of 33 to 1390 days. An ABR of 35 was added to account for the periods when these people were on prophylaxis.

After ROCTAVIAN, your doctor will monitor your lab results and talk to you about whether you can stop prophylaxis and whether you should start prophylaxis again if stopped. In ROCTAVIAN clinical studies, patients who did not respond to treatment or lost response to treatment were able to resume prophylaxis.
Your doctor will also discuss with you whether and how you should treat for any surgeries, procedures, injuries, or bleeds.
*ROCTAVIAN worked for 73% (82/112) of people in the rollover population and 59% (13/22) of people in the directly enrolled population throughout the 4-year follow-up period.
†Prophylaxis is defined as the ongoing use of Factor VIII or another treatment to prevent bleeds.
‡The 4-year follow-up period began 5 weeks or more after administration and consisted of a median follow-up of 4 years with a range of 1.7 to 4.6 years.
§Data were collected for 6 months, and those results were annualized.
Get safety information from the ROCTAVIAN clinical study.
*At approval, ROCTAVIAN had the longest and largest Phase 3 clinical study.
Connect with a BioMarin representative.
Do not take ROCTAVIAN if you:
What is the most important information I should know about ROCTAVIAN?
ROCTAVIAN may cause serious side effects during the infusion and afterward:
What should I tell my doctor before I get ROCTAVIAN?
Talk to your doctor about the following:
What should I avoid after taking ROCTAVIAN?
What are the possible side effects of ROCTAVIAN?
What other information should I know before getting ROCTAVIAN?
Talk to your doctor about the potential risks and benefits of ROCTAVIAN. Whether a patient experiences a benefit or not, the risks discussed here and with your doctor still apply.
These are not all the possible side effects of ROCTAVIAN. Talk to your doctor for medical advice about side effects. You may report side effects to BioMarin Pharmaceutical Inc. at 1-866-906-6100 or FDA at 1-800-FDA-1088.
Please see additional safety information in the Prescribing Information and Patient Information.
What is ROCTAVIAN?
ROCTAVIAN is a one-time gene therapy used for the treatment of adults with severe hemophilia A who do not have antibodies to the virus, AAV5 which is determined by a blood test. ROCTAVIAN uses a modified virus, called a vector, to deliver a working copy of the Factor VIII gene to liver cells to enable your body to produce clotting factor on its own, which helps the blood to clot and prevents or reduces the occurrence of bleeding. The modified virus does not contain viral DNA and does not cause disease in humans.